
New Drug Designations - December 2024
Shots:
- PharmaShots' designation report provides a concise overview of the latest drug designations by major regulatory authorities, including the FDA, EMA, MHLW and NMPA
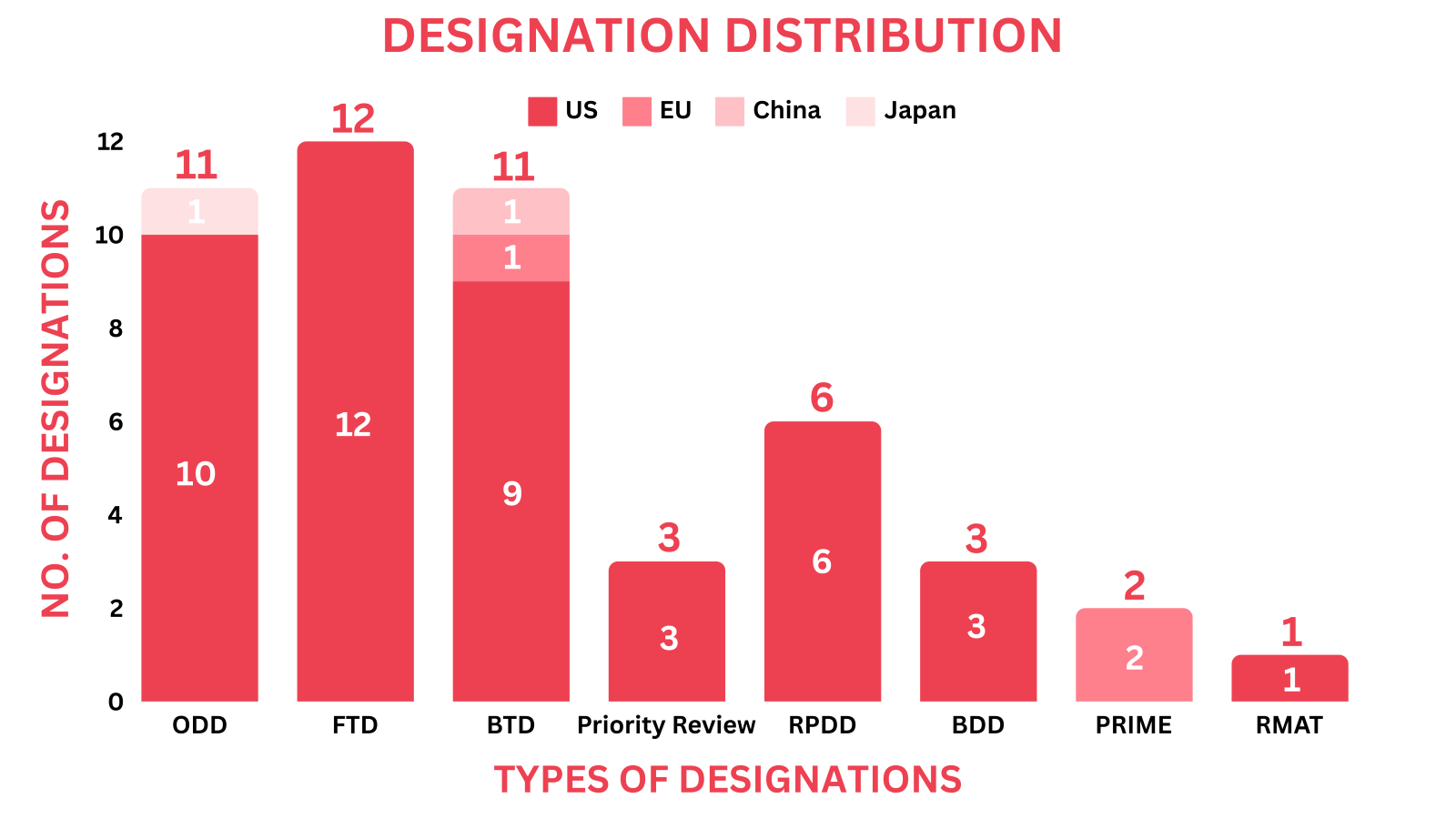
- The December 2024 report covers designations granted to 43 drugs and 3 devices, encompassing 13 small molecules, 10 biologics, 7 cell and gene therapies & 3 medical devices among others
- Significant trends this month show, Nobias Therapeutics’ NB-001 secured the US FDA’s Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation (RPDD) to treat DiGeorge syndrome

Ersodetug - Biologic
.png)
- The US FDA has granted ODD to ersodetug for the treatment of hypoglycemia caused by tumor Hyperinsulinism (HI)
- The company is expecting to initiate a P-III trial assessing ersodetug in tumor HI patients in 2025 with real-world data exhibited through an expanded access program
- Ersodetug is a fully human mAb that opposes the effects of over-activated insulin receptors by binding to its unique allosteric site
NB-001 - Antivirals
.png)
- The US FDA has granted ODD & RPDD to NB-001 for the treatment of patients with neuropsychiatric symptoms related to 22q11.2 deletion syndrome (DiGeorge syndrome/22q11DS)
- NB-001 is currently under investigation in a P-II trial assessing NB-001 vs PBO in 22q11DS patients with top-line results presented at CNS Annual Meeting, 2023
- NB-001 is a mGluR modulator that disrupts neuronal signaling that relieves neuropsychiatric symptoms
Crofelemer - Small molecule
.png)
- The US FDA has granted ODD to crofelemer for treating diarrhea in cholera patients
- Additionally, the company intends to pursue ODD & a Tropical Disease Priority Review Voucher for NP-300 to treat diarrhea in cholera patients
- Crofelemer is an investigation drug candidate currently in 3 Investigator-Initiated Trial (IIT) PoC & 2 P-II studies for SBS-IF ± MVID in the US/ EU/ Middle East/ North Africa regions. The first dosing for the same is expected in Dec’24 & Q1’25, whereas IIT PoC results are expected in Q2’25
ST316 - Peptides
.png)
- The US FDA has granted ODD to ST316 for the treatment of patients with familial adenomatous polyposis (FAP)
- ST316 is currently in the P-II study of the P-I/II trial (ST316-101) assessing ST316 with relevant SoC & multiple lines of treatment in CRC patients, while the P-I was the dose escalation study in patients having advanced solid tumors with Wnt/β-catenin signaling pathway abnormalities including colorectal cancer (CRC)
- ST316 (β-catenin & BCL9 antagonist) selectively inhibits Wnt/β-catenin signaling pathway responsible for FAP & >80% of CRCs in patients
Umbilical Cord Outer Lining Stem Cells (UCSLs) - Cell Therapy
.png)
- The US FDA has granted ODD to UCSLs programs for the treatment of Polymyositis (PM) & Dermatomyositis (DM) patients
- The therapy demonstrated positive results in P-I study with significant clinical improvements; favorable safety & efficacy plus a probable decrease in the need for steroidal therapies. The initiation of P-II/III trials is expected in Q1’2025
BRC-002 - Cannabinoid
.png)
- The US FDA has granted ODD to BRC-002 for the treatment of complex regional pain syndrome (CRPS) that is currently under investigation in the IIT P-I study
- The company plans to initiate patient enrollment for the P-II study of BRC-002 by the YE’25 & seeks FDA input on the development plan
- BRC-002 is a novel, botanically derived oral cannabinoid therapy designed to treat pain and co-morbidities of CRPS, featuring a proprietary blend of major and minor cannabinoids.
Azeliragon - Small Molecule
.png)
- The US FDA has granted ODD to azeliragon for the treatment of patients with brain metastasis from breast cancer
- Azeliragon (oral, QD) inhibits RAGE interactions with its ligands (incl. HMGB1 & S100 proteins) & is under investigation for brain metastasis, glioblastoma, breast cancer, pancreatic cancer, & hospitalized pneumonia patients
- Originally developed by vTv Therapeutics for Alzheimer’s disease, Cantex has licensed worldwide rights to Azeliragon
AVG-002 – Gene Therapy
.png)
- The US FDA has granted ODD to AVG-002 for neonatal SP-B deficiency, following the grant of RPDD in Nov’24. AVG-002 is anticipated to enter clinical development & filing is expected by 2028
- The Preclinical data showed increased survivability in SP-B deficient murine model with a single dose of AVG-002 plus recovery of lung histology & function in disease-induced tissue after AVG-002 treatment
- AVG-002, an inhalable gene therapy, is developed using AlveoGene’s InGenuiTy platform that utilizes a pseudo-typed lentiviral vector to efficiently deliver the gene directly to the alveolar region of the lung
Manufactured Red Blood Cells – Cell Therapy
.png)
- The US FDA has granted ODD & RPDD to manufactured RBCs (mRBCs) for use in chronic transfusion to treat sickle cell patients
- The company is advancing its validation of mRBCs cGMP manufacturing for clinical studies & anticipates initiating the trials by 2027, post-completion of IND-enabling activities
VGT-1849A - antisense oligonucleotide
.png)
- The US FDA has granted ODD to VGT-1849A for the treatment of patients with polycythemia vera (PV)
- VGT-1849A is a selective ASO-based JAK2 inhibitor with the potential to decrease JAK2V617F-driven pathogenic signaling, thereby reducing malignant proliferation & survivability of hematopoietic cells
Sparsentan – Small Molecule
.png)
- The Japanese MHLW has granted ODD to sparsentan, being investigated under P-III study in Japan for treating IgA nephropathy. It was already approved by the US FDA under the brand name Filspari for slowing kidney function among adults in Sep 2024
- Renalys secured its development & commercialization rights from Travere Therapeutics across Japan, South Korea, Taiwan, Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand & Vietnam as per an agreement signed in Jan 2024
- Filspari (QD, oral) is a non-immunosuppressive medication for IgAN that blocks endothelin-1 and angiotensin II pathways to reduce glomerular injury

PT217 - Biologic
.png)
- The US FDA has granted FTD to PT217 for treating metastatic de novo or treatment-emergent neuroendocrine prostate cancer (NEPC)
- PT217 is being investigated under P-I/II (SKYBRIDGE) trial for its safety, tolerability, pharmacokinetics and preliminary efficacy in patients with advanced or refractory cancers with DLL3 expression and another P-I study in China (CTR20242720)
- PT217 is a first-in-class bispecific antibody targeting DLL3 & CD47 and being developed for SCLC & neuroendocrine carcinoma (NEC), including NEPC. Phanes has also partnered with Roche to study it in combination with atezolizumab
AdAPT-001 - Immunotherapy
.png)
- The US FDA has granted FTD to AdAPT-001 + nivolumab/atezolizumab for the treatment of r/r advanced or metastatic soft tissue sarcoma (STS) post disease progression after at least 1L of therapy
- The Designation was supported by data from P-I/II trials assessing AdAPT-001 as monotherapy or in combination with PD-1/PD-L1 inhibitors showed PFS of ~8.5mos. in patients with STS & other tumors.
- The FTD for AdAPT-001 was granted due to its potential to sensitize STS tumors to checkpoint inhibitors (nivolumab or atezolizumab), even in cases where prior treatment failed or was not received due to low tumor mutation burden (TMB) and T-cell inflamed gene expression profiles (GEP), which predict non-response.
- AdAPT-001 is an oncolytic adenovirus-delivered TGF-βR inhibitor that neutralizes TGFβ locally to decrease T cell function & sensitize STS tumors to checkpoint inhibitors
Fluzone & Flublok + Novavax - Vaccine

- The US FDA has granted FTDs to two combination vaccine candidates, Fluzone (influenza protein-based trivalent vaccine) + Novavax (COVID-19 vaccine) & Flublok + Novavax, for preventing influenza and COVID-19 infections in subjects (50+ yrs.)
- Fluzone High-Dose and Flublok outperformed standard-dose flu vaccines in preventing influenza and reducing flu-related hospitalizations in older adults. The Novavax COVID-19 vaccine has shown better tolerability as a booster and high efficacy as a primary vaccination in P-III studies
- Sanofi has launched 2 P-I/II studies to assess the safety and immune response of combination vaccines to prevent influenza A, influenza B & COVID-19
- NCT06695117: Combines Fluzone High-Dose (TIV-HD) with the Novavax COVID-19 vaccine for individuals aged 50+ yrs.
- NCT06695130: Combines Flublok (RIV3) with the Novavax COVID-19 vaccine for the same age group
IMM-1-104 – Small Molecule
.png)
- The US FDA has granted FTD to IMM-1-104 for the treatment of unresectable or metastatic NRAS-mutant melanoma patients progressed or intolerable to PD-1/PD-L1 checkpoint inhibitors
- IMM-1-104 (QD) inhibits MAPK pathway to stimulate RAS activity that impacts cancer cells selectively & is investigated in a P-IIa study for advanced solid tumors including melanoma
LPCN 1148 – Small Molecule
.png)
- The US FDA has granted FTD to LPCN 1148 for the treatment of sarcopenia in decompensated cirrhosis patients
- LPCN 1148 was subjected to a recent PoC P-II study in decompensated cirrhosis patients, showing improved sarcopenia & related clinical outcomes
- LPCN 1148 (oral) contains testosterone dodecanoate (androgen receptor agonist) & utilizes multimodal approach to manage cirrhosis & related comorbidities
NX-5948 – Small Molecule
.png)
- The US FDA has granted FTD to NX-5948 for the treatment of r/r Waldenstrom’s macroglobulinemia (WM) in patients who received at least 2L of treatment, including a BTK inhibitor
- The designation is followed by P-I data evaluating the safety & efficacy of NX-5948 & the company is enrolling WM patients in a P-Ib (expansion cohort) trial with results expected in 2025
- NX-5948 is a novel oral small molecule that degrades BTK proteins through cereblon E3 ligase complex without affecting other cereblon neo-substrates
BGC101 – Cell Therapy
.png)
- The US FDA has granted FTD to BGC101 for the treatment of Critical Limb Threatening Ischemia (CLTI) patients to prevent amputations, disease progression & relieve pain
- BGC101 is under investigation in a P-II study, which completed patient recruitment & is being conducted in the US, EU & Israel
- BGC101 utilizes BioGenCell's TRACT platform to stimulate tissue regeneration in the damaged limb by developing personalized cell therapies through the immune & stem cells obtained from the patient
SC291 – Cell Therapy
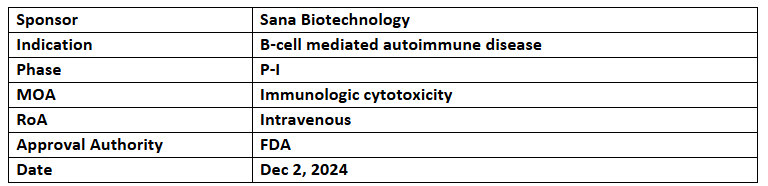
- The US FDA has granted FTD to SC291 for the treatment of r/r SLE, including extrarenal lupus & lupus nephritis
- SC291 is being evaluated in the GLEAM trial in B-cell mediated autoimmune disease patients (including lupus nephritis, extrarenal lupus, plus ANCA-associated vasculitis) with ongoing enrolment & initial results expected in 2025
- SC291, an allogeneic CAR T cell therapy, is developed using Sana’s hypoimmune platform that targets CD19 protein expressed on the surface of B cells
R289 – Small Molecules
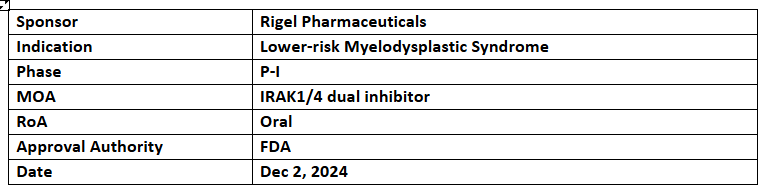
- The US FDA has granted FTD to R289 in patients with previously treated transfusion dependent lower-risk myelodysplastic syndrome (LR-MDS)
- The P-Ib study is currently assessing the safety, tolerability, pharmacokinetics & preliminary efficacy of R289 among patients with r/r lower-risk MDS, with recruitment underway
- R289 (prodrug of R835) is an IRAK1/4 dual inhibitor that works by blocking inflammatory cytokine production in response to TLR and IL-1R signaling. Dysregulation of these pathways contributes to inflammatory conditions
LP-184 – Small Molecule
.png)
- The US FDA has granted FTD to LP-184 for the treatment of Triple Negative Breast Cancer (TNBC) in patients, following the grant of FTD to treat Glioblastoma in Oct 2024
- The preclinical data showed tumor regression (107-141%) in both PARPi (PARP inhibitor) resistant & PARPi sensitive tumors across 10 TNBC PDX models. Recent preclinical data was presented at Immuno-Oncology Summit 2024
- LP-184 is being assessed in a P-Ia trial to evaluate its safety and tolerability in a broad range of solid tumor patients, including TNBC
- LP-184 leverages Lantern's AI platform, RADR & is activated into its cytotoxic form by the enzymatic action of Prostaglandin Reductase 1 (PTGR1), to produce anti-tumor effects
CRB-701 - Biologic
.png)
- The US FDA has granted FTD to CRB-701 for the treatment of patients with r/r metastatic cervical cancer
- CRB-701 is being assessed in a 3-part, P-I trial evaluating its safety, PK & efficacy to treat advanced solid tumors linked to elevated Nectin-4. Enrollment of the dose escalation part has been completed which will be conducted in the US & Europe, initial data is expected to be reported in Q1’25
- CRB-701 (SYS6002) is an ADC with MMAE as the cytotoxic payload & site-specific, cleavable linker to target Nectin-4 expression on the cancer cells
LBT-SA7 – Vaccine
.png)
- The US FDA has granted FTD to LBT-SA7 to prevent skin and soft tissue infections caused by Staphylococcus aureus
- LBT-SA7 (multivalent toxoid vaccine) will be assessed in a P-I trial in the US, to demonstrate its safety & immunogenicity in 130 subjects (18 - 50yrs.), with initial results expected in H2’25

ATX101- Peptides
.png)
- The US FDA has granted BTD to ATX101 for treating adults with post-surgical pain following total knee replacement surgery
- The designation was based on a dose-ranging P-II exploratory study of ATX101 vs bupivacaine (SoC) in 112 subjects, showing sustained pain relief for up to 2wks., decreased opioid use & associated side effects plus improved functional activities & satisfaction for up to 60 days. A registrational P-IIb trial in 200 subjects is planned in 2025 across the US
- ATX101 is an investigational formulation of bupivacaine (intracellular sodium ion channel blocker) and a biopolymer indicated for relieving pain post-total knee arthroplasty (TKA)
Datopotamab Deruxtecan (Dato-DXd) - Biologic
.png)
- The US FDA has granted BTD to Dato-DXd for treating locally advanced or metastatic EGFR-mutated NSCLC in adults progressed on an EGFR tyrosine kinase inhibitor (TKI) and Pt-based CT
- Designation was based on P-II (TROPION-Lung05) & P-III (TROPION-Lung01) studies, with the results highlighted at ESMO 2024
- Daiichi Sankyo and AstraZeneca have filed a new BLA seeking accelerated approval for treating locally advanced or metastatic EGFR-mutated NSCLC in adults who received prior systemic therapies, including an EGFR-directed therapy
Tolebrutinib – Small Molecule
.png)
- The US FDA has granted BTD to tolebrutinib for treating adults with non-relapsing secondary progressive multiple sclerosis (nrSPMS)
- The designation was based on a P-III (HERCULES) study assessing the safety & efficacy of tolebrutinib vs PBO to treat nrSPMS that showed a 31% delay in 6mos. confirmed disability progression onset, with 10% vs 5% having confirmed disability improvement
- Liver enzyme elevations (>3xULN) occurred in 4.1% vs 1.6%, with 0.5% showing ALT >20xULN within 90 days. Most cases resolved without intervention, and increased monitoring has mitigated serious liver issues
- Regulatory filings of tolebrutinib are being finalized in the US & prepared in the EU. In addition, the P-III (PERSEUS) study in primary progressive MS is underway, with results expected in H2’25
Jemperli - Biologic
.png)
- The US FDA has granted BTD to Jemperli for locally advanced mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) rectal cancer.
- The designation was provided based on a P-II study (conducted with Memorial Sloan Kettering Cancer Center) in dMMR rectal cancer patients (n=42), showing a 100% clinical CR in all, without any evidence of tumors on MRI, endoscopy, PET scan, or digital rectal exam
- Furthermore, sustained cCR was observed at a median follow-up of 26.3mos. in the first 24 of them and is underway to assess the patients; safety profile aligned with known data without grade 3+ AEs. GSK’s registrational P-II (AZUR-1) trial is ongoing to validate this data
Trodelvy - Biologic

- The US FDA has granted BTD to Trodelvy for treating adult with extensive-stage small cell lung cancer (ES-SCLC) whose disease has progressed on Pt-based CT
- The designation was based on P-II (TROPiCS-03) study ES-SCLC cohort, showing promising antitumor activity in both Pt-resistant (PR) & sensitive (PS) disease as a 2L treatment for ES-SCLC. Gilead further plans to start a P-III trial in ES-SCLC patients
- P-III trials will assess Trodelvy alone and in combinations across various cancers, in collaboration with academic, industry & global partners
- Trodelvy (sacituzumab govitecan-hziy) is a first-in-class ADC targeting Trop-2, a cell surface antigen overexpressed in over 90% of breast and lung cancers
Sacituzumab Tirumotecan - Biologic
.png)
- The US FDA has granted BTD to sac-TMT for treating advanced or metastatic non-squamous EGFR-mutated (exon 19 deletion/exon 21 L858R) NSCLC in patients progressed post TKI & Pt-based CT
- Designation was supported by P-II expansion part of P-I/II study and two P-II trials of sac-TMT in EGFR-mutated NSCLC patients treated with at least two prior therapies, was highlighted at ASCO 2023
- Merck is developing sac-TMT alone & combined with Keytruda under 10 global P-III trials for solid tumors, including TroFuse-004 (sac-TMT vs CT) & TroFuse-009 (sac-TMT vs doublet CT) in previously treated EGFR-mutated NSCLC. Kelun Biotech holds the Greater China rights for sac-TMT
Zorevunersen - Antisense oligonucleotides (STK-001)
.png)
- The US FDA has granted BTD to zorevunersen for treating Dravet syndrome with a confirmed mutation, not associated with gain-of-function, in the SCN1A gene
- The P-I/IIa and open label studies have depicted significant & sustained seizure reduction as well as improved cognition & behavior. The drug was well tolerated
- Stoke Therapeutics is in talks with regulators about a P-III study of zorevunersen and will provide an update by YE’24
- Zorevunersen is an antisense oligonucleotide (ASO) and a potential first disease-modifying therapy for Dravet syndrome. It aims to restore physiological NaV1.1 protein levels by upregulating the non-mutant SCN1A gene, reducing seizures and associated non-seizure comorbidities
SER-155
.png)
- The US FDA has granted BTD to SER-155 for reducing bloodstream infections (BSIs) in adults, receiving allogeneic hematopoietic stem cell transplant (allo-HSCT) to treat hematological malignancies
- Designation was based on P-Ib study of SER-155 vs PBO, showing a 77% reduction in bacterial BSIs (10% vs 42.9%), shorter period of antibiotic use (9.2 vs 21.1 days) and reduced febrile neutropenia & gastrointestinal pathogen domination. Seres is looking for a collaboration to support its development
- SER-155 (oral) is a biotherapeutic aimed at preventing bacterial bloodstream infections and AMR-related outcomes in allo-HSCT patients by decolonizing GI pathogens and improving immune tolerance
Tobevibart - Biologic and Elebsiran – RNA Therapy
.png)
- The US FDA & the EMA have granted BTD & PRIME Designation, respectively, to tobevibart & elebsiran for the treatment of chronic hepatitis delta (CHD) patients
- The designations were based on a P-II (SOLSTICE) trial evaluating tobevibart ± elebsiran in CHD patients with results presented at AASLD The Liver Meeting. The P-III (ECLIPSE) trial assessing tobevibart & elebsiran in CHD is to initiate in H1’25
- Tobevibart is a mAb targeting the hepatitis B surface antigen. It aims to block hepatitis B and delta virus entry into hepatocytes and lower circulating viral and subviral particles
- Elebsiran is a siRNA therapy targeting hepatitis B virus (HBV) RNA transcripts to reduce hepatitis B surface antigen production. It shows potential for direct antiviral activity against both HBV and hepatitis delta virus (HDV)
Orpathys and Tagrisso – Small Molecules
.png)
- The China’s NMPA has granted BTD to Orpathys + Tagrisso combination for treating locally advanced or metastatic EGFR+ NSCLC with MET amplification after progressing on EGFR inhibitor therapy
- The combination is being assessed under P-III (SACHI) study for its safety & efficacy in comparison with Pt-based doublet-CT (pemetrexed + cisplatin/carboplatin) to treat locally advanced or metastatic EGFR+ NSCLC
- The 1EP includes PFS by investigator evaluation while other EPs are PFS by IRC evaluation, OS, ORR, DoR, DCR, time to response (TTR) & safety
- Orpathys is a potent, selective oral MET TKI with proven clinical activity in advanced solid tumors. Tagrisso (osimertinib) is a third-generation, irreversible EGFR-TKI with proven efficacy in NSCLC, including CNS metastases

Imfinzi - Biologic
.png)
- The US FDA has accepted & granted priority review to sBLA of Imfinzi for treating muscle-invasive bladder cancer (MIBC), with decision anticipated in Q2’25. Further submissions are under review across the EU, Japan & other regions
- sBLA was based on P-III (NIAGARA) study assessing perioperative Imfinzi + neoadj. CT followed by Imfinzi adj. vs CT alone treatment in 1063 patients with MIBC, with data presented at ESMO 2024 & published in the NEJM
- Interim analysis showed reduced disease progression, recurrence, or death risk by 32% (mEFS: not reached vs 46.1mos.), 67.8% vs 59.8% were event free at 2yrs. Imfinzi also reduced death risk by 25% (mOS not reached), 82.2% vs 75.2% were alive at 2yrs.
- Imfinzi (durvalumab) is a mAb that binds to the PD-L1 protein that blocks PD-L1 interactions, helping the immune system counter tumor evasion
Avutometinib + Defactinib
.png)
- The US FDA has accepted & granted priority review (PDUFA: Jun 30, 2025) to NDA of avutometinib + defactinib for treatment-experienced KRAS-mutated recurrent LGSOC adults, under accelerated approval pathway. Launch is expected in mid-2025
- NDA was based on P-I (FRAME) trial & P-II (RAMP 201) trial of avutometinib (3.2mg, BIW) ± defactinib (200mg, BID) in LGSOC patients, identifying the combination as go-forward treatment in Part A; Parts B & C will assess the regimen’s safety & efficacy & Part D will explore low-dose regimen
- P-II showed significant ORRs & good tolerability. The P-III (RAMP 301) study will confirm results for this indication & is recruiting LGSOC patients regardless of KRAS mutations for indication expansion
- Avutometinib is an oral RAF/MEK clamp that inhibits MEK1/2 kinase activities while inducing inactive MEK-RAF complexes. Defactinib is an oral inhibitor of focal adhesion kinase (FAK) and proline-rich tyrosine kinase-2 (Pyk2)
Taletrectinib – Small Molecule
.png)
- The US FDA has accepted the NDA of taletrectinib (next-generation ROS1 TKI) and granted priority for treating advanced ROS1+ NSCLC, with the decision anticipated on Jun 23, 2025
- The submission was based on pooled data from ongoing P-II [TRUST-I (China) & TRUST-II (global)] studies assessing the safety & efficacy of taletrectinib monotx. for treating advanced NSCLC, with the data highlighted at ESMO 2024
- Pooled analysis showed cORR of 89% & intracranial cORR of 77% with mDoR of 44mos. & mPFS of 46mos. in TKI-naive patients (n=160). In TKI-pretreated 113 patients, cORR was 56% & 62% for G2032R mutations, with intracranial cORR of 66%, mDoR of 17mos. & mPFS of 10mos.
- Taletrectinib is an oral, next-generation ROS1 inhibitor for advanced ROS1+ NSCLC. It is potent, CNS-active, and selective, aiming to improve treatment outcomes in ROS1-driven lung cancer

101-PGC-005 - Antivirals
.png)
- The US FDA has granted RPDD to 101-PGC-005 (‘005) for treating systemic juvenile idiopathic arthritis (sJIA) flares
- 101-PGC-005 is currently being investigated under P-III study to treat ARDS induced by COVID-19 across 9 sites in India
- 005 is an investigational Type IA prodrug of dexamethasone designed to selectively target CD206+ macrophages
NB-001
.png)
- The US FDA has granted ODD & RPDD to NB-001 for the treatment of patients with neuropsychiatric symptoms related to 22q11.2 deletion syndrome (DiGeorge syndrome/22q11DS)
- NB-001 is currently under investigation in a P-II trial assessing NB-001 vs PBO in 22q11DS patients with top-line results presented at CNS Annual Meeting, 2023
- NB-001 (mGluR modulator) disrupts neuronal signaling that relieves neuropsychiatric symptoms
THIO – Small Molecule
.png)
- The US FDA has granted RPDD to THIO for treating pediatric type diffuse high-grade gliomas (PDHGG)
- Alongside RPDD, the drug has also been designated with ODD for hepatocellular carcinoma (HCC), small cell lung cancer (SCLC) and glioblastoma
- THIO (6-thio-dG or 6-thio-2’-deoxyguanosine) is a telomere-targeting candidate that is being investigated for NSCLC
HORA-PDE6b – Gene Therapy
.png)
- The US FDA has granted RPDD to HORA-PDE6b for treating inherited retinal dystrophy (IRD) caused by PDE6b gene mutations
- HORA-PDE6b is being studied under P-I/II trial for its safety & efficacy, with the 24mos. follow-up data highlighted at ARVO 2024
- EyeDNA is seeking for accelerated approval across the US & the EU for patients with this indication
- HORA-PDE6b, an AAV5-based gene therapy, delivers a functional PDE6b gene to the subretinal space, restoring protein synthesis in photoreceptors and potentially halting retinal degeneration in PDE6b-deficient patients
Relutrigine – Small Molecule
.png)
- The US FDA has granted RPDD to relutrigine for treating Dravet syndrome. Relutrigine is being assessed under P-II (EMBOLD) study for its safety & efficacy in reducing seizures. Findings from SCN2A and SCN8A patients in cohort 1 were highlighted at AES 2024
- Study depicted a 46% reduction in PBO-adjusted monthly motor seizure during double-blind, 30% were seizure free, 77% reduction in median seizure rate during long-term extension & notable improvements in alertness, communication & seizure severity
- Praxis has begun recruitment for second, registrational cohort for SCN2A and SCN8A patients, with topline data anticipated in H1’26
- Relutrigine is an investigational first-in-class small molecule being developed for the treatment of developmental and epileptic encephalopathy (DEE). It acts as a preferential inhibitor of persistent sodium current, which plays a key role in seizure symptoms associated with SCN2A-DEE and SCN8A-DEE
Manufactured Red Blood Cells
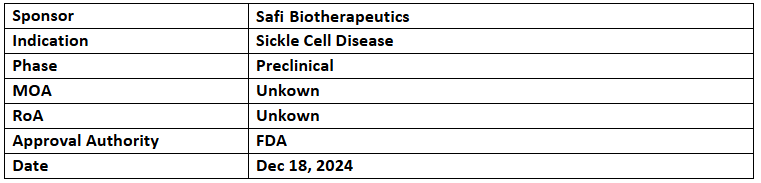
- The US FDA has granted ODD & RPDD to manufactured RBCs (mRBCs) for use in chronic transfusion to treat sickle cell patients
- Safi is advancing its validation of mRBCs cGMP manufacturing for clinical studies & anticipates initiating them by 2027, post-completion of IND-enabling activities

Magnetic EEG Resonance Therapy (MeRT) System
.png)
- The US FDA has granted BDD to Magnetic EEG Resonance Therapy (MeRT) system as an adjunctive treatment of post-traumatic stress disorder (PTSD)
- The MeRT system uses a proprietary algorithm to personalize Transcranial Magnetic Stimulation (TMS) treatment based on an individual's brain wave data
FieldForce Ablation System
- The US FDA has granted BDD to FieldForce Ablation System for sustained monomorphic scar-related ventricular tachycardia (VT), which makes it eligible for Total Product Life Cycle Advisory Program (TAP) Pilot
- The FieldForce Ablation System consists of a single-point contact force PFA catheter with proprietary FieldBending tech, enabling precise targeted lesions and large-volume transmural lesions in the ventricle
MeMed Severity Test
.png)
- The US FDA has granted BDD to MeMed Severity test to manage suspected acute infections and suspected sepsis
- The test assesses various proteins in the blood sample using advanced host-response technology and identifies the risk of suspected acute infection deteriorating to severe outcomes within 72 hrs. or death within 14 days through ML

Tobevibart and Elebsiran
.png)
- The US FDA & the EMA have granted BTD & PRIME Designation, respectively, to tobevibart & elebsiran for the treatment of chronic hepatitis delta (CHD) patients
- The designations were based on a P-II (SOLSTICE) trial evaluating tobevibart ± elebsiran in CHD patients with results highlighted at AASLD The Liver Meeting. The P-III (ECLIPSE) trial assessing tobevibart & elebsiran in CHD is to initiate in H1’25
- Tobevibart is a mAb targeting the hepatitis B surface antigen. It aims to block hepatitis B and delta virus entry into hepatocytes and lower circulating viral and subviral particles
- Elebsiran is a siRNA therapy targeting hepatitis B virus (HBV) RNA transcripts to reduce hepatitis B surface antigen production. It shows potential for direct antiviral activity against both HBV and hepatitis delta virus (HDV)
GSK’227 - Biologic
.png)
- The EMA has granted PRIME designation to GSK5764227 (GSK’227 or HS-20093) based on preliminary data from P-I (ARTEMIS-001) study conducted out by Hansoh Pharma
- The P-I trial is assessing safety, tolerability & anti-tumor activity of GSK’227 in locally advanced or metastatic solid tumors (incl. ES-SCLC) patients (n=>200), with data highlighted at WCLC 2024. A global P-I trial has begun to support its registrational filing
- GSK secured GSK’227’s exclusive worldwide rights (excl. mainland mainland China, Hong Kong, Macau & Taiwan) from Hansoh as per an agreement b/w them
- GSK'227 is a B7-H3-targeted ADC, for treating relapsed extensive-stage SCLC

AAV2-hAQP1 – Gene Therapy
- The US FDA has granted RMAT designation to AAV2-hAQP1 for treating Grade 2/3 radiation-induced xerostomia (RIX)
- Designation was based on P-I (AQUAx) study showing significantly improved patient-reported outcomes & saliva production without treatment-related SAEs or DLTs. Results were highlighted at AAOM 2024
- The P-II (AQUAx2) trial currently assesses AAV2-hAQP1 vs PBO continues recruitment and dosing across the US, UK & Canada
References
- Rezolute
- Jaguar Health
- Sapience Therapeutics
- Biopharmaceutical Research Company
- Cantex Pharmaceuticals
- AlveoGene
- Vanda Pharmaceuticals
- Renalys
- Phanes Therapeutics
- Nurix Therapeutics
- Rigel
- Lantern Pharma
- Sana Biotechnology
- Corbus Pharmaceuticals
- Lipocine
- LimmaTech
- Sanofi
- EpicentRx
- Immuneering
- Daiichi Sankyo
- GSK
- Gilead
- Merck
- Stoke Therapeutics
- Seres Therapeutics
- Vir Biotechnology
- HUTCHMED
- AstraZeneca
- Verastem
- Nuvation Bio
- MAIA Biotechnology
- EyeDNA Therapeutics
- Praxis Precision Medicines
- MeMed
- MeiraGTx Holdings
- BusinessWire
- PRWeb
- Globe Newswire
- PR Newswire
- EIN Presswire
- Google News
Related Post: New Drug Designations - November 2024

An avid reader and a dedicated learner, Prince works as a Content Writer at PharmaShots. Prince possesses an exceptional quality of breaking down the barriers of words by simplifying the terms in digestible chunks to make content readable and comprehensible. Prince likes to read books and loves to spend his free time learning and upskilling himself.














